 发布时间:2022/03/22
发布时间:2022/03/22产品介绍
CRM197是一种将52位的甘氨酸替换为谷氨酸的单一突变的无毒性的白喉毒素突变体(文献1,2),致使白喉毒素酶活性位点发生改变,从而不能对细胞产生毒性作用,但在抗原性和免疫原性上仍和天然白喉毒素保持一致。CRM197是一种研究明确的蛋白质,作为多糖和半抗原的载体使其具有免疫原性。它被用作一种载体蛋白,用于许多已经被批准的疫苗,比如脑膜炎球菌、乙型流感嗜血杆菌和肺炎球菌感染的结合疫苗。
Pfenex的CRM197是一种使用Pfenex Expression Technology平台在荧光假单胞菌中表达的重组形式。CRM197是结合疫苗的理想载体蛋白或免疫佐剂。通过物理化学特性和体内研究表明,Pfenex的 CRM197在结构和功能上与白喉棒状杆菌产生的突变蛋白相同。目前的研究和开发领域包括免疫疗法和抗成瘾性疫苗。可提供满足任何临床研究或商业化生产要求的研究等级或者cGMP等级的规格。自1990年以来,CRM197已被商业应用于预防性疫苗中。
2020年Pfenex被Ligand Pharmaceuticals收购,现在CRM197也被称为PeliCRM。
产品特点
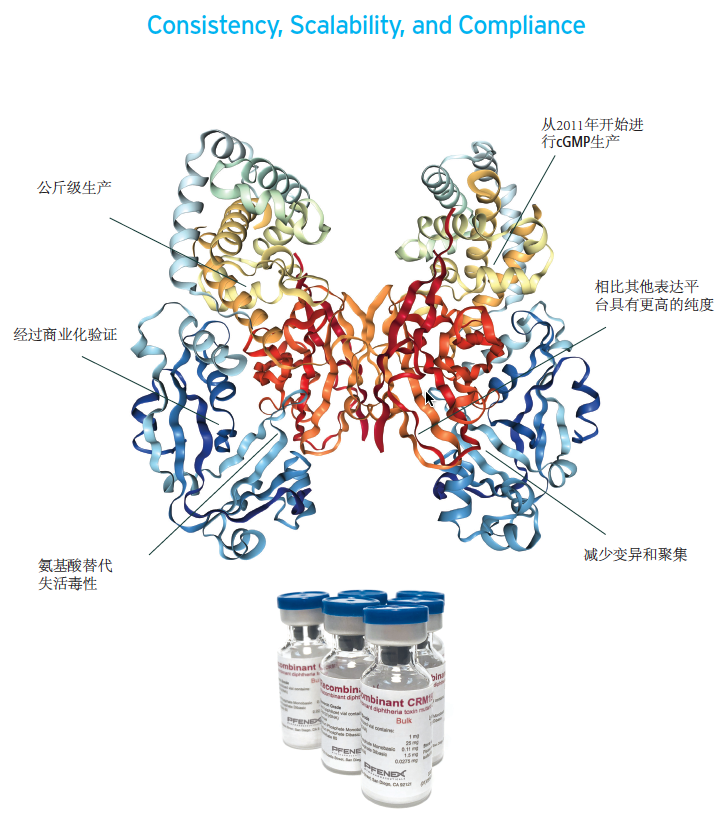
l避免了在病原微生物中缓慢,复杂和昂贵的生产
l与其他的表达系统获得的CRM197相比,Pnefex的纯度更高
l提供研究用和cGMP级的重组CRM197载体蛋白(3)
l从研究等级(mg级)到cGMP级(kg级)的产品和工艺保持一致性
l加速疫苗研发进入临床,是疫苗开发和商业化的经济有效的关键材料来源
l可提供生物制品生产主文件(Master File),用于支持FDA临床监管申报和美国以外区域的同等机构申报
l生产工艺经过针对Pneumosil疫苗开发的WHO预审。
(3) 重组CRM197(bulk)产品是根据现行cGMP生产规范和ICH Q7和ICH Q11(视情况而定)指导原则制造的,重组CRM197(bulk)作为活性物质起始材料,用于最终用户进一步制造合适的原料药和/或药品。
使用方法
收到CRM197冻干粉后请保存在2-8°C。
使用前,用无菌去离子水将冻干的CRM197无菌复原至4 mg/mL的浓度 。
配置好的重组CRM197应在2-8°C下储存。
产品参数
来源: 荧光假单胞菌重组表达的 CRM197
产品分子量: 58.4 kDa
纯度:>95% CRM197 by SE-HPLC or RP-HPLC or SDS-PAGE
内毒素: <100 EU/mg of protein by LAL method
二聚物:<5%
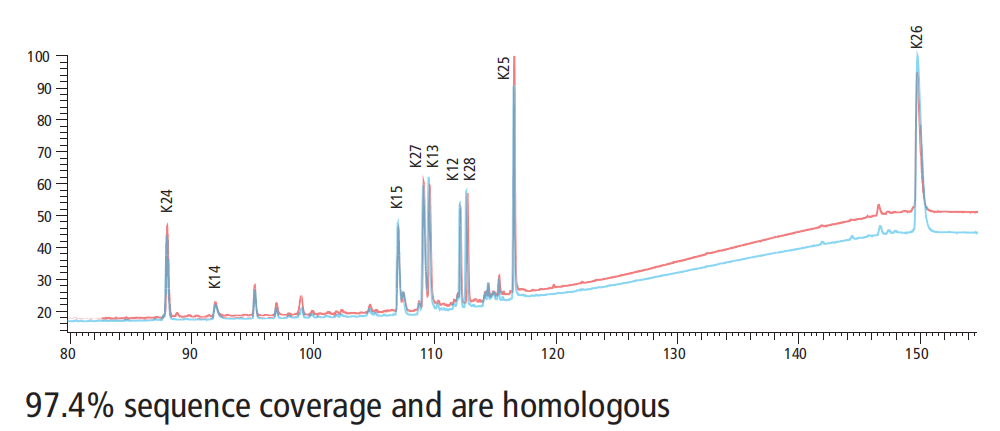
数据展示
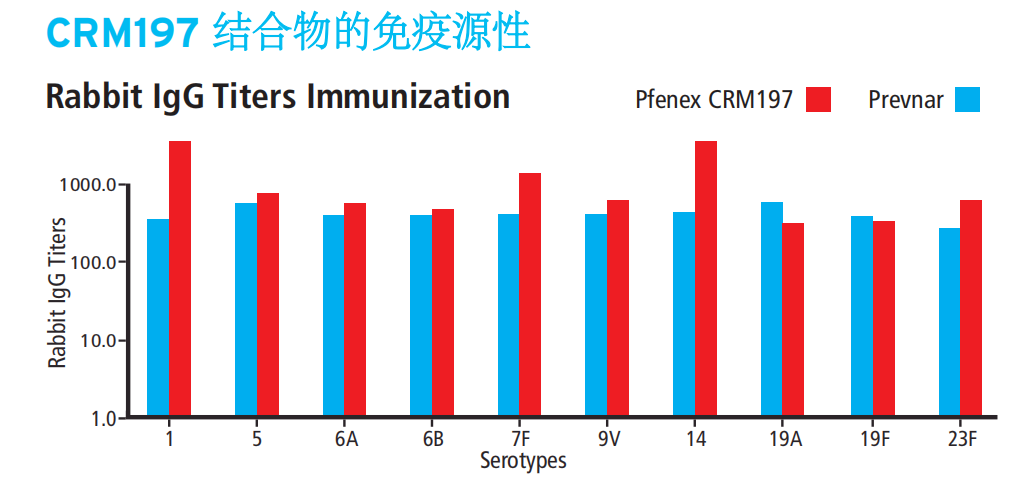

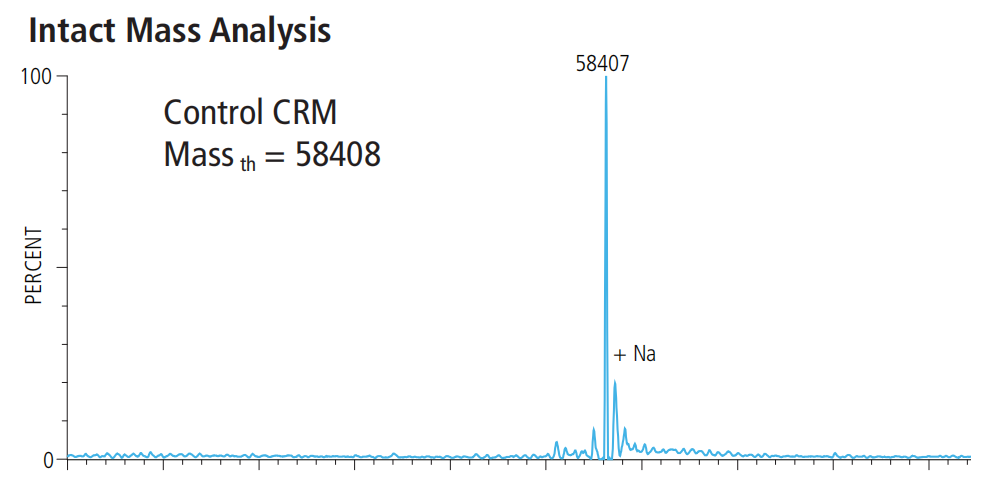
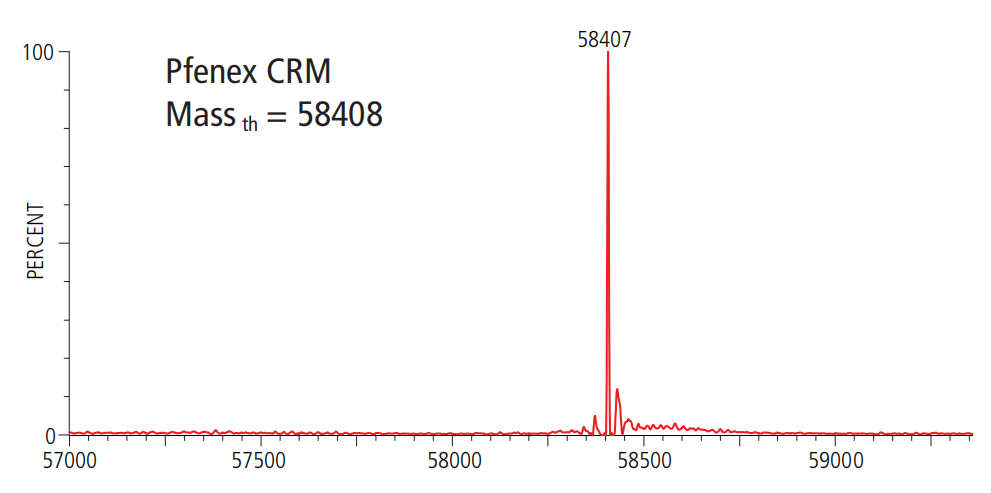
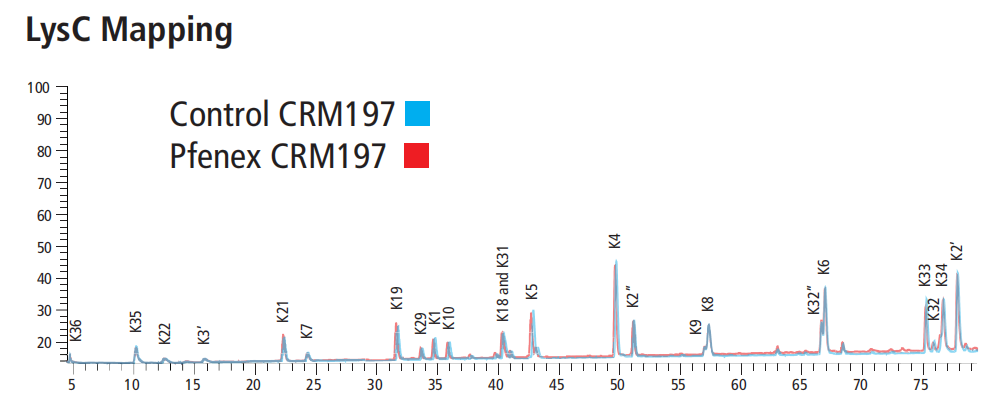
相关文献
1. Giannini, G., Rappuoli, R., and Ratti, G. Nucleic Acids Res. 12: 4063-4069 (1984)
2. Mekada, E., and Uchida, T. J. Biol. Chem. 260: 12148-12153 (1985)
The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM45 and CRM197.
Nucleic Acids Research | By G Giannini, R Rappuoli, and G Ratti | May 25, 1984
Abstract: The amino-acid sequences of two diphtheria toxin-related, non-toxic proteins, CRM45 and CRM197 , were deduced from the complete sequence of their genes: tox 45 and tox 197. CRM45 lacks the last 149 C-terminal amino-acid residues, but is otherwise identical to diphtheria toxin: a single C—-T transition introduces an “ochre” (TAA) termination signal in tox 45, after the codon for threonine-386. A single G—-A transition was also found in tox 197, leading to the substitution of glycine-52, present in the wild-type toxin, with glutamic acid in CRM197 . This aminoacid change is responsible for the loss of the NAD:EF2 ADP-ribosyltransferase activity in CRM197 , due most probably to an alteration of the NAD+ binding site.
Identification of diphtheria toxin receptor and a nonproteinous diphtheria toxin-binding molecule in Vero cell membrane
Journal of Cell Biology | By: Mekada, E., and Uchida, T. J. | August 1, 1988
Abstract: Two substances possessing the ability to bind to diphtheria toxin (DT) were found to be present in a membrane fraction from DT-sensitive Vero cells. One of these substances was found on the basis of its ability to bind DT and inhibit its cytotoxic effect. This inhibitory substance competitively inhibited the binding of DT to Vero cells. However this inhibitor could not bind to CRM197, the product of a missense mutation in the DT gene, and did not inhibit the binding of CRM197 to Vero cells. Moreover, similar levels of the inhibitory activity were observed in membrane fractions from DT-insensitive mouse cells, suggesting the inhibitor is not the DT receptor which is specifically present in DT-sensitive cells. The second DT-binding substance was found in the same Vero cell membrane preparation by assaying the binding of 125I-labeled CRM197. Such DT-binding activity could not be observed in membrane preparation from mouse L cells. From competition studies using labeled DT and CRM proteins, we conclude that this binding activity is due to the surface receptor for DT. Treatment of these substances with several enzymes revealed that the inhibitor was sensitive to certain RNases but resistant to proteases, whereas the DT receptor was resistant to RNase but sensitive to proteases. The receptor was solubilized and partially purified by chromatography on CM- Sepharose column. Immunoprecipitation and Western blotting analysis of the partially purified receptor revealed that a 14.5-kD protein is the DT receptor, or at least a component of it.